CONTENTS
Chapter 1
DEVELOPMENT OF NATURAL PRODUCTS
Robert Verpoorte
Introduction
Plant Secondary Metabolites
Development of New Natural Products
Production and Quality Control
Conclusions
References
Chapter 2
LEGAL SYSTEMS AND REGULATIONS
Willem K Scholten
Introduction
Differences in Legal Systems
Points to Consider with Herbal Regulations
National and Regional Regulations
Worldwide Harmonization
References
Chapter 3
OVERVIEW OF GLOBAL REGULATORY STATUS
KK Bhutani and HR Jadhav
Introduction
Regulatory Status
Summary and Conclusion
Chapter 4
REGULATORY CONTROLS ON PHYTOMEDICINES
VB Desai, S Badami, Giriraj T Kulkarni and B Suresh
Introduction
Standardization of Phytomedicines
Standards for Herbal Dosage Forms
Standardization of Herbal Products - Indian Scenario
Current Regulatory Controls on Phytomedicines
Conclusion
References
Chapter 5
HERBAL MEDICINE REGULATIONS IN RUSSIA
Severtsev VA, Makarov VG, Shikov AN and Pozharitskaya ON
Introduction
Herbal Medicine Industry in Russia
Regulating Production of Herbal Medicines in Russia
Licensing for Manufacture of Herbal Medicines
Quality Standards of Herbal Medicines in Russia
Discussions
References
Chapter 6
PRODUCING QUALITY BOTANICALS THROUGH GMP
Rajeev K Sharma
Herbal Drugs and ISM Pharmaceuticals
Quality of Herbal Drugs
Basic Perception of Quality
Quality Variation Factors in Herbal Drugs
Historical Background
Status and Applicability of GMP Regulations
Objectives of GMP
Salient Features of GMP Under Schedule - T For ISM Drugs
Components of GMP (Schedule -T)
GMP Provisions: A Bird Eye View
Quality Control Vis-À-Vis Quality Assurance
Quality Control of Herbal Drugs
Methodologies/Standardization of Herbal Drugs
References
Chapter 7
GMP REQUIREMENTS IN INDIAN SYSTEMS OF MEDICINE
Pulok K Mukherjee
Introduction
Regulation and Registration of Herbal Medicines
Need for GMP for Botanicals
GMP in Indian Systems of Medicine
General Requirements for GMP of ASU Drugs
Raw Materials for ASU Drugs
Quality Assurance And Quality Control
Documentation in The GMP for ASU Drugs
Self-Inspection and Quality Audits
Implementation of GMP for ASU Drugs
Conclusion
References
Chapter 8
GUIDANCE ON GMP FOR PHYTOMEDICINES
JPS Kohli
General
Personnel
Buildings and Facilities
Sanitation
Equipment
Maintenance
Computer Systems Validation
Calibration
Warehousing
Quality Manual
Site Master File
References
Chapter 9
FORMULATION TECHNIQUES IN AYURVEDA
KS Jayashree
Introduction
Evam Prakruthihi (Identification)
Eavam Guna (Qualities)
Eavam Prabhavam (Empirical Knowledge)
Asmin Deshejathaha (Ecology)
Asmin Rituvevam Grahitham (Season of Collection)
Anaya Matraya Nihitam (Posology)
Asmin Vyadhou Prayuktam (Suitable for this Disease)
Etavamtham Dhoshamapakarshati (Effect on the Doshas)
Shodhana (Purification Procedures)
Tests for Perfect Processing
Conclusions
References
Chapter 10
QUEST FOR GMP FOR THE PRODUCTION OF BOTANICALS
Pulok K Mukherjee and BP Saha
Introduction
Botanicals and their Assessment
Good Agricultural Practices
Good Practices in Collection of Plant Materials
Primary Processing of Herbal Products
Documentation Required
Other Guidelines for Quality Assurance of Herbal Drugs
References
Chapter 11
DEVELOPMENT OF AYURVEDIC MEDICINE
D Shanthkumar Lucas
Introduction
Ayurvedic Education and Research
Drug Development Through Ayurveda
Chapter 12
MANUFACTURING AND QUALITY CONTROL OF AYURVEDIC AND HERBAL PREPARATIONS
J Singh, GD Bagchi and SPS Khanuja
Introduction
Processing of Ayurvedic Drugs
Bioprospection, Drug Development and Pharmacogenomics
Legislature in India
Good Manufacturing Practices
Standardization and Quality Control
Chemical and DNA Fingerprinting of Herbal Drugs
Safety and Efficacy Evaluation
References
Chapter 13
ENSURING SAFE USE OF HERBAL DRUGS
MK Chattopadhyay
Introduction
Problems
Toxicity
Quantitative and Qualitative Variation
Interaction with Other Drugs
Adulteration
Paucity of Information
Regulations in Different Countries
Conclusion
References
Chapter 14
QUALITY AND SAFETY OF BOTANICALS
K Sadasivan Pillai and V Amalan Stanley
Introduction
Process Validation
Safety Evaluation of Drugs
References
Chapter 15
ANALYSIS OF SOUTH PACIFIC HERBAL DRUGS
Subramaniam Sotheeswaran
Introduction
Herbal Drugs of Commercial Importance
Future Directions
Conclusion
References
Chapter 16
QUALITY CONTROL OF BOTANICALS CONTAINING ESSENTIAL OILS
A Zaenglein
Introduction
Parameters and Methods
GMP Specifications
Problem Definition
Experimentals
Results and Discussion
Conclusions
References
Chapter 17
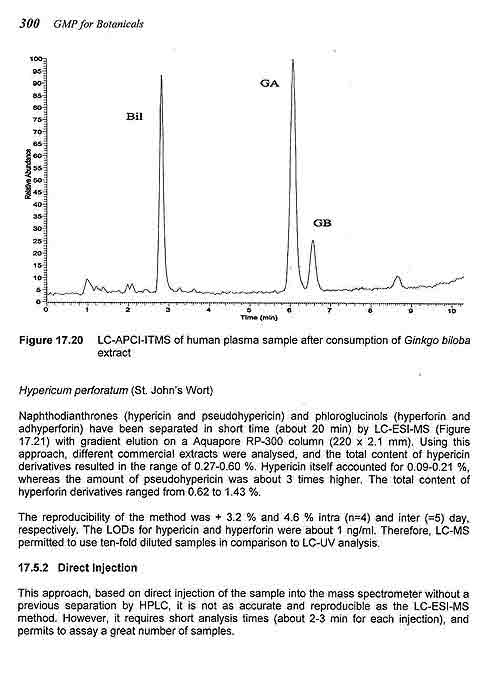
MASS SPECTROMETRY APPLICATIONS IN HERBAL MEDICINE ANALYSIS
Pierluigi Mauri and Piergiorgio Pietta
Introduction
Instrumental
Quality Control of Medicinal Plants
Qualitative Analysis
Quantitative Analysis
Medicinal Plant Analysis by MS
Conclusions
References
Chapter 18
CAPILLARY ELECTROPHORESIS IN HERBAL MEDICINE ANALYSIS
Piergiorgio Pietta and Claudio Gardana
Introduction
Analysis of Medicinal Plants
Experimental Procedure
Conclusions
References
Chapter 19
PHARMACOLOGICAL EVALUATION
A Subramonium
Introduction
Problems Facing Development of GMP
Pharmacological Evaluation of Herbal Drugs
Pharmacological Standards for Herbal Drugs
Conclusions
References
Chapter 20
CLINICAL TRIALS OF HERBAL MEDICINES
Carmen Tamayo
Introduction
Basic Concepts
Classification of RCTs
RCTs Methodology Concepts
Herbal Medicine Concepts
Herbal Medicine Research Methodology
Issues in Clinical Research of Herbal Medicines
Good Protocol Characteristics
Clinical Trial Development
Challenges of Herbal Medicine's RCTs
IND Requirements in the Evaluation of Herbal Products
References
Chapter 21
ROLE OF BIOASSAYS IN DEVELOPMENT OF NATURAL PRODUCTS
Amit Agarwal and Prasanth D Souza
Introduction
Classification of Bioassays
Use of Bioassays in Quality Control
Use of Bioassays to Assess Combinational Activity
Use of Bioassays in Stability Testing
Overview
References