CONTENTS
Introduction
Introduction to the Second Edition
Preface to the First Edition
1 DESIGNING EFFECTIVE SOPs
1.1 Introduction and Purpose of SOPs
1.2 Benefits of SOPs
1.3 Types of SOPs
1.4 Contents of a Typical SOP
1.5 Level of Detail
1.6 Writing Style
1.7 SOP Development
1.7.1 Create/Edit
1.7.2 Review
1.7.3 Approve
1.7.4 Publish
1.7.5 Distribute
1.7.6 Archive
1.8 SOP Format
1.8.1 Title Page
1.8.2 Table of Contents
1.8.3 Text
1.9 Transition to Electronic SOPs
1.9.1 Too Many Documents
1.9.2 Over-complex Documents
1.9.3 Inappropriate Format
1.9.4 Management Systems
1.10 Advantages of Electronic SOPs
1.11 System Requirements
1.12 Structure of eSOP System
1.12.1 Audit Critical Activities
1.12.2 Authenticate Users
1.12.3 Keep Track of Who Has Seen What
1.12.4 Validation Support
1.13 Future of eSOP
2 ELECTRONIC MANUFACTURING – eMANUFACTURING
2.1 Understanding the ‘e’ in the eManufacturing
2.1.1 Electronic Records
2.1.2 Requirements for Electronic Records
2.1.3 Risk Based Approach to Future
2.2 Purpose of eManufacturing
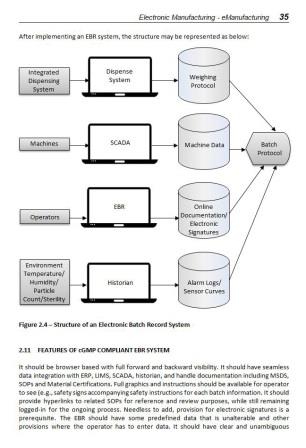
2.3 Introduction to Electronic Batch Records (EBR)
2.4 Benefits of Electronic Batch Records (EBR)
2.5 Plant Efficiencies
2.5.1 Impact of QC Lab on Batch Cycle Time
2.6 Management of Manufacturing Data
2.7 User Requirement Specification (URS)
2.8 Vendor Selection
2.9 Qualification and Validation of Software
2.10 Evolution from Paper to EBR
2.11 Features of cGMP Compliant EBR System
2.12 Process Historian for Management of Manufacturing Data
2.13 Enterprise Resource Planning for Pharmaceuticals
2.13.1 Implementation
2.13.2 ERP for Small and Medium Business (SMB)
2.13.3 Pharmaceutical Applications of ERP
2.14 IT Infrastructure for Pharmaceutical Industry
2.15 SOP for Standard Operating Procedures
OSD ORAL SOLIDS MANUFACTURING SECTION
OSD-CM Cleaning and Maintenance
OSD-CM-01 Cleaning and Assembly of Compression Machine
OSD-CM-02 Cleaning and Assembly of Strip Sealing Machine
OSD-CM-03 Cleaning of Autoloader
OSD-CM-04 Cleaning of Capsule Inspection cum Polishing Machine
OSD-CM-05 Cleaning of Conventional Coating Pan
OSD-CM-06 Cleaning of Double Cone Blender
OSD-CM-07 Cleaning of Dry Syrup Powder Filling Machine
OSD-CM-08 Cleaning of Dust Exhausts
OSD-CM-09 Cleaning of Fluid Bed Dryer
OSD-CM-10 Cleaning of Manual Capsule Filling Machine
OSD-CM-11 Cleaning of Mass Mixer
OSD-CM-12 Cleaning of Multimill
OSD-CM-13 Cleaning of Octagonal Blender
OSD-CM-14 Cleaning of Planetary Mixer
OSD-CM-15 Cleaning of Polishing Pan
OSD-CM-16 Cleaning of Prefilter of Fluid Bed Drier
OSD-CM-17 Cleaning of Rapid Mixer Granulator
OSD-CM-18 Cleaning of Semi-Automatic Capsule Filling Machine
OSD-CM-19 Cleaning of Sifter
OSD-CM-20 Cleaning of Spray Gun
OSD-CM-21 Cleaning of Tablet Department
OSD-CM-22 Cleaning of Tray Dryer
OSD-CM-23 Cleaning of Vibratory Tablet De-Duster
OSD-CM-24 Cleaning of Wurster Coater Drier
OSD-CM-25 Validation of Cleaning Procedure
OSD-CM-26 Housekeeping – General
OSD-CM-27 Housekeeping - Pest Control and Disinfection
OSD-CM-28 Housekeeping - Storing Used Mop
OSD-CM-29 Housekeeping - Use of Floor Wiper
OSD-CM-30 Housekeeping - Vacuum Cleaner Operation and Cleaning
OSD-CM-31 Housekeeping Check Points for Capsule Department
OSD-CM-32 Housekeeping in Tablet Compression/Packaging Area
OSD-CM-33 Inspection of Punches and Dies
OSD-CM-34 Operation of Punch Polishing Machine
OSD-CM-35 Setup and Operation of Tablet Punch Polisher
OSD-OP Operations
OSD-OP-01 Operation of Autoloader
OSD-OP-02 Operation of Blister Packing Machine
OSD-OP-03 Operation of Capsule Inspection cum Polishing Machine
OSD-OP-04 Operation of Conventional Coating Pan
OSD-OP-05 Operation of Double Cone Blender
OSD-OP-06 Operation of Fluid Bed Dryer
OSD-OP-07 Operation of Multimill
OSD-OP-08 Operation of Octagonal Blender
OSD-OP-09 Operation of Paste Preparation Vessel
OSD-OP-10 Operation of Pouch Packing Machine
OSD-OP-11 Operation of Pre-Sifting
OSD-OP-12 Operation of Punch Polishing Machine
OSD-OP-13 Operation of Rapid Mixer Granulator
OSD-OP-14 Operation of Semi-Automatic Capsule Filling Machine
OSD-OP-15 Operation of Spheronizer
OSD-OP-16 Operation of Strip Packing Machine
OSD-OP-17 Operation of Tray Drier
OSD-OP-18 Operation of Wurster Coater Dryer
OSD-OP-19 Sifting of Powdered Excepient/Active Material
OSD-OP-20 Startup Tablet Process
OSD-OP-21 Strip Packing - Sealing Test
OSD-MG Management
OSD-MG-01 Charge Hand Over Between the Shifts
OSD-MG-02 Destruction of Granules Bulk/Tablets
OSD-MG-03 Disposal Operation in Tablet Department
OSD-MG-04 Documentation - Authorization for Change
OSD-MG-05 Documentation - Cancellation of Batch Number
OSD-MG-06 Documentation - Maintenance of Records and Reports
OSD-MG-07 Documentation Controls
OSD-MG-08 Guidelines for Inspection Checkers
OSD-MG-09 Guidelines for Polishing of Punches & Dies
OSD-MG-10 In-Process Checks on the Packaging Line
OSD-MG-11 Issues and Receipts of Punch Set
OSD-MG-12 New Equipment
OSD-MG-13 New Punch Set
OSD-MG-14 Oil Application Procedure for Compression Tools and Machine
OSD-MG-15 Packing Slip on Final Pack (On Shipper)
OSD-MG-16 Policy on Replacement of Punches
OSD-MG-17 Procedure for Closing the Capsule/Tablet Department
OSD-MG-18 Procedure for Destruction of Blister Packs
OSD-MG-19 Procedure for Opening the Tablet/Capsule Department
OSD-MG-20 Proper Attire for Production Areas
OSD-MG-21 Raw Material Dispensing Procedure
OSD-MG-22 Reconciliation of Printed Packaging Materials
OSD-MG-23 Safety Appliances
OSD-MG-24 Sieve Analysis for Lubricated Granules
OSD-MG-25 Stopping the Operation in Coating Section
OSD-MG-26 Stopping the Operation in Compression Section
OSD-MG-27 Stopping the Operation of Granulation Section
OSD-MG-28 Storage Condition for Coating Raw Material
OSD-MG-29 Storage of In-Process Tablets and Granules
OSD-MG-30 Storage of Tablet Compression Machine When Not in Use
OSD-MG-31 Storage of Tablet Compression Tooling
OSD-MG-32 Tablet Compression - In Process Controls
OSD-MG-33 Tablet Compression - Tooling
OSD-MG-34 Tabletting Operations
OSD-MG-35 Washing of Feet
OSD-MG-36 Washing of New Finger Bag Made From Epitropic Cloth
OSD-MG-37 Work in Process Standards
OSD-MG-38 Working Procedure for Capsule Department
OSD-MG-39 Working Procedure for Packing Department
OSD-CB Calibration
OSD-CB-01 Checking Punch Height
OSD-CB-02 Guidelines for Calibration of Equipment
OSD-TN Training
OSD-TN-01 Training Program for Supervisors
OSD-TN-02 Training Program for Workmen
ORL ORAL LIQUIDS MANUFACTURING SECTION
ORL-CM Cleaning and Maintenance
ORL-CM-01 Cleaning of Bottle Labeling Machine
ORL-CM-02 Cleaning of Filling Machine
ORL-CM-03 Cleaning of SS Water Transfer Pipe
ORL-CM-04 Cleaning of Tanks, Accessories, Utensils and Stirrers
ORL-CM-05 Cleaning Validation of Compounding Tank
ORL-CM-06 Cleaning Validation of SS Liquid Transfer Pipe
ORL-CM-07 Cleaning/Replacement of Filters for Window Air Conditioners
ORL-CM-08 Housekeeping in Liquid Oral Compounding Area
ORL-CM-09 Housekeeping in Liquid Oral Packing Area
ORL-OP Operations
ORL-OP-01 Leakage Test of Filled Bottles
ORL-OP-02 Operation and Cleaning of Filter Press
ORL-OP-03 Operation of Bottle Labeling Machine
ORL-OP-04 Operation of Colloid Mill
ORL-OP-05 Operation of Rotary Bottle Washing Machine
ORL-MG Management
ORL-MG-01 Destruction Certificate for In-Process Goods
ORL-MG-02 Fill Volume Determination
ORL-MG-03 Finished Goods Storage and Handling
ORL-MG-04 Finished Goods Verification
ORL-MG-05 In-Process Control for Liquid Orals
ORL-MG-06 Liquid Oral Filling
ORL-MG-07 Overprinting of Packaging Supplies
ORL-MG-08 Precautions for Compounding and Cleaning
ORL-MG-09 Reconciliation of Rejected and Excess Packing Materials
ORL-MG-10 Reprocessing of Products
ORL-CB Calibration
ORL-CB-01 Calibration of Compounding/Holding Vessels
SSD SEMI SOLIDS MANUFACTURING SECTION
SSD-CM Cleaning and Maintenance
SSD-CM-01 Cleaning and Assembly of Preparation Tank
SSD-CM-02 Cleaning and Assembly Procedure - Silverson Mixer
SSD-CM-03 Cleaning and Assembly Procedure - Tube Filling Machine
SSD-CM-04 Cleaning and Sanitization of Ointment Department
SSD-CM-05 Cleaning of Bulk Storage Drum
SSD-CM-06 Cleaning of Compounding Kettle
SSD-CM-07 Cleaning of Flooring and Wall Surfaces
SSD-CM-08 Cleaning of SS Melting Tank
SSD-CM-09 Cleaning of Utensils, Scrappers and Scoops
SSD-CM-10 Cleaning Validation of Equipment
SSD-CM-11 Housekeeping in Ointment Department
SSD-MG Management
SSD-MG-01 Controlling Microbial Contamination
SSD-MG-02 Destruction of Bulk from Tube Filling Machine
SSD-MG-03 Destruction of Rejected Goods and Packaging Materials
SSD-MG-04 In Process Controls - Manufacturing
SSD-MG-05 In Process Controls - Packaging
SSD-MG-06 In Process Fill Weight Determination
SSD-MG-07 Line Clearance
STR STERILE LIQUIDS MANUFACTURING SECTION
STR-CM Cleaning and Maintenance
STR-CM-01 Cleaning & Disinfection of Sterile Area Class 10,000 and Class 100
STR-CM-02 Cleaning & Disinfection of the Sterile Area Class 10000 after Media Filling
STR-CM-03 Cleaning and Assembly of Rubber Stopper Washing Machine
STR-CM-04 Cleaning and Maintenance of Autoclave
STR-CM-05 Cleaning and Storage of Equipment for Sterile Products
STR-CM-06 Cleaning of Area after Power Failure
STR-CM-07 Cleaning of Bronze Sintered Filter (for Compressed Air)
STR-CM-08 Cleaning of Compounding/Filtration Tanks and Homogenizer
STR-CM-09 Cleaning of Holding Vessels
STR-CM-10 Cleaning of Labeling Machines
STR-CM-11 Cleaning of Membrane Filter Holder
STR-CM-12 Cleaning of Micro Metallic SS Spargers/Filters
STR-CM-13 Cleaning of Non Sterile Manufacturing Areas
STR-CM-14 Cleaning of the Autoclave Chamber
STR-CM-15 Cleaning of the Header Tank (DM Water Reservoir)
STR-CM-16 Cleaning of the Stirrer
STR-CM-17 Cleaning of Vial Sealing Machine
STR-CM-18 Cleaning of Vial/Ampoule Inspection Machine
STR-CM-19 Cleaning Procedure for Ampoule Filling and Sealing Machine
STR-CM-20 Cleaning Procedure for Distilled Water Storage Tank
STR-CM-21 Cleaning Procedure for DM Water Surge Tank
STR-CM-22 Cleaning Procedure for Vials /Ampoules Washing Machine
STR-CM-23 Cleaning Validation for Equipment
STR-CM-24 DM Water Filter Cleaning
STR-CM-25 Inspection, Cleaning and Maintenance of Sterilizing Ovens
STR-CM-26 Routine Washing of Micro Metallic Spargers
STR-CM-27 Rubber Stopper Treatment
STR-CM-28 Washing and Assembly of Membrane Filter Holder
STR-CM-29 Washing of the Batch Filtration Tubing
STR-CM-30 Washing of Vial /Ampoule Filling Assemblies and Accessories
STR-CM-31 Washing, Assembly and Sterilization of Filling Assemblies and Accessories (Regular and after Media Filling)
STR-CM-32 Washing, Assembly & Sterilization of Filling Assemblies and Accessories
STR-CM-33 Washing, Wrapping and Sterilization of Gloves
STR-OP Operations
STR-OP-01 Air Sampling Using Centrifugal Air Sampler
STR-OP-02 Aseptic Fill Validation
STR-OP-03 Aseptic Filling
STR-OP-04 Measurement of Dissolved Oxygen Content
STR-OP-05 Measurement of Head Space Oxygen
STR-OP-06 Operation of Ampoule Filling Machine
STR-OP-07 Operation of Ampoule Inspection Machine
STR-OP-08 Operation of Autoclave
STR-OP-09 Operation of Labeling Machine
STR-OP-10 Operation of Particle Counter
STR-OP-11 Operation of the Distillation Still
STR-OP-12 Operation of UV Intensity Meter
STR-OP-13 Operation of Vial Filling Machine
STR-OP-14 Operation of Vial Inspection Machine
STR-OP-15 Operation of Vial Labeling Machine
STR-OP-16 Oxygen Level Determination in Filling Tank
STR-OP-17 Preparation of Disinfectant Solution for Sterile Area Cleaning
STR-OP-18 Preparation of Gum for Labeling Vials and Ampoules
STR-OP-19 Preparation of Solution for pH Adjustment
STR-MG Management
STR-MG-01 Assigning Expiry Date to Drug Products
STR-MG-02 Assigning Expiry Dates to Sterilized Items
STR-MG-03 Check List of Jobs before Start-up and After Shutdown
STR-MG-04 Checking of Vial Sealing
STR-MG-05 Clean Room - Condition Monitoring (Air Changes)
STR-MG-06 Clean Room Procedures - General Guidelines
STR-MG-07 Control and Placement Plans of Biological Monitors
STR-MG-08 De-Ionized / Distilled Water Control Record
STR-MG-09 Destruction Guidelines of Vials/Ampoules at Various Stages of Production
STR-MG-10 Destruction of Batch - Sterile Products
STR-MG-11 Destruction of Rejected In-Process Goods and Packaging Materials
STR-MG-12 Destruction of Rejected Vials/Ampoules
STR-MG-13 Disposal of Rejected and Left Over Printed Packing Material
STR-MG-14 DM Water Line - Use of UV Bank
STR-MG-15 DM Water Plant Regeneration
STR-MG-16 DM Water Re-Circulation
STR-MG-17 Electronic Weighing Machine
STR-MG-18 Finished Goods at Bonded Store Room
STR-MG-19 Fumigation of Sterile Area
STR-MG-20 Good Manufacturing Practices
STR-MG-21 Handling of Terminally Sterilized and Non Sterilized Ampoules
STR-MG-22 In Process Controls
STR-MG-23 Inspection of Garments Used in the Sterile Filling Room
STR-MG-24 Inspection of Injectables for Human Use
STR-MG-25 Inspection of Sterile Preparations
STR-MG-26 Master Monitor for Sterile Area Operations
STR-MG-27 Monitoring of Flow Direction in Sterile Area
STR-MG-28 Monitoring of HEPA Filters
STR-MG-29 Monitoring of Positive Pressure, Air Temperature and Relative Humidity
STR-MG-30 Nitrogen Retention Test in Holding Vessel/Filling Vessel
STR-MG-31 Packing Start Up
STR-MG-32 Policy for Changing Filters in Non Sterile Area
STR-MG-33 Policy for HEPA Filter Changes
STR-MG-34 Procedure for Entry of Major Equipment in Sterile Area
STR-MG-35 Procedure for Entry through Dry Heat Sterilizer
STR-MG-36 Procedure for Entry to Sterile Area - Gowning
STR-MG-37 Procedure for Integrity and Pressure Hold Test of Membrane Filter
STR-MG-38 Procedure for Integrity Testing of Membrane Filters Used for Gases
STR-MG-39 Procedure for Leak Rate Test - Autoclave
STR-MG-40 Procedure for Leak Testing of Filled Ampoules
STR-MG-41 Procedure for Line Clearance
STR-MG-42 Procedure for Line Clearance - Labeling
STR-MG-43 Procedure for Material Entry into Sterile Area
STR-MG-44 Procedure for Packing (Sterile Products)
STR-MG-45 Procedure for Swab Test
STR-MG-46 Procedure for Vial / Ampoule Preparation
STR-MG-47 Reconciliation of Printed Packing Materials
STR-MG-48 Remedies for High Microbial Counts
STR-MG-49 Self Inspection
STR-MG-50 Servicing Policy - Clean Air Systems & Devices Monitoring
STR-MG-51 Sterile Room - Precautions and Restrictions
STR-MG-52 Sterility Testing of Products
STR-MG-53 Sterilization Cycles - Autoclave
STR-MG-54 Sterilization Cycles - Dry Heat Sterilizer
STR-MG-55 Storage Conditions for In-Process Goods
STR-MG-56 Unloading of Sterile Charge of Autoclave
STR-MG-57 Unloading of Sterile Charge of Dry Heat Sterilizer
STR-MG-58 Validation of Dry Heat Sterilizer
STR-MG-59 Validation of the Autoclave
STR-MG-60 Yield and Reconciliation Controls
STR-CB Calibration
STR-CB-01 Calibration and Validation Program
STR-CB-02 Calibration of 12 Point Temperature Indicator
STR-CB-03 Calibration of Dial Type Hygrometer
STR-CB-04 Calibration of Instruments Installed on Autoclave
STR-CB-05 Calibration of Magnehelic Gauge
STR-CB-06 Calibration of Oxygen Analyzer
STR-CB-07 Calibration of Pressure Gauge Used for Membrane Integrity Test
STR-CB-08 Calibration of Thermocouple of Temperature Measuring Devices for Autoclave
STR-CB-09 Calibration of Thermocouple of Temperature Measuring Devices of Dry Heat Sterilizer
STR-CB-10 Calibration of Volume of Compounding Tanks
STR-TN Training
STR-TN-01 Training Program - Design and Format
STR-TN-02 Training Program for Autoclave
STR-TN-03 Training Program for Dry Heat Sterilizer
STR-TN-04 Training Program for Janitors
STR-TN-05 Training Program for Laminar Air-Flow
STR-TN-06 Training Program for Membrane Filter Holder
STR-TN-07 Training Program for Personal Hygiene
STR-TN-08 Training Program for Production Supervisor
STR-TN-09 Training Program for Sterile Stoppering
STR-TN-10 Training Program for the Workmen
STR-TN-11 Training Program for Vial Sealing
QCL QUALITY CONTROL LABORATORY SECTION
QCL-CM Cleaning and Maintenance
QCL-CM-01 Cleaning of Glassware for Analytical Work
QCL-CM-02 Cleaning Procedure for Microbiology Lab
QCL-CM-03 Servicing of Fume Chamber in QC Department
QCL-OP Operations
QCL-OP-01 Operation and Calibration of Bacteriological Incubator
QCL-OP-02 Operation and Calibration of Balance (Electronic)
QCL-OP-03 Operation and Calibration of Balance (Platform)
QCL-OP-04 Operation and Calibration of Balance (Single Pan)
QCL-OP-05 Operation and Calibration of BOD Incubator
QCL-OP-06 Operation and Calibration of Bursting Strength Tester
QCL-OP-07 Operation and Calibration of Colony Counter
QCL-OP-08 Operation and Calibration of Compound Microscope
QCL-OP-09 Operation and Calibration of Disintegration Test Apparatus
QCL-OP-10 Operation and Calibration of Dissolution Test Apparatus
QCL-OP-11 Operation and Calibration of FTIR Apparatus
QCL-OP-12 Operation and Calibration of Friability Test Apparatus
QCL-OP-13 Operation and Calibration of IR Moisture Balance
QCL-OP-14 Operation and Calibration of Melting Point Apparatus
QCL-OP-15 Operation and Calibration of Oven
QCL-OP-16 Operation and Calibration of pH Meter
QCL-OP-17 Operation and Calibration of Photo-Fluorometer
QCL-OP-18 Operation and Calibration of Polarimeter
QCL-OP-19 Operation and Calibration of Refractometer
QCL-OP-20 Operation and Calibration of Spectrophotometer
QCL-OP-21 Operation and Calibration of Vernier Caliper
QCL-OP-22 Operation and Calibration of Zone Reader
QCL-OP-23 Operation and Validation of Autoclave
QCL-OP-24 Operation and Validation of Laminar Flow
QCL-OP-25 Operation of Bulk Density Apparatus
QCL-OP-26 Operation of Centrifuge Machine
QCL-OP-27 Operation of Hardness Tester
QCL-OP-28 Operation of Molybdenum Kit
QCL-OP-29 Operation of UV Cabinet
QCL-OP-30 Operation of Vacuum Oven
QCL-OP-31 Operation and Cleaning of Milli-Q Gradient Water Purification System
QCL-OP-32 Operation and Cleaning of Alltech Model 3000 Solvent Recycler
QCL-OP-33 Operation, Cleaning and Calibration of MS/MS Detector Model Micromass Quattro Ultima
QCL-OP-34 Operation, Cleaning and Calibration of Rotary Shaker
QCL-OP-35 Operation and Performance Verification of Heto Ultra Deep Freezer
QCL-OP-36 Operation and Calibration of Ultrasonic Cleaner
QCL-OP-37 Operation and Calibration of Electro-Lab Dissolution Tester with Auto Sampler
QCL-OP-38 Procedure for Documentation of Analytical Data
QCL-OP-39 Operation and Performance Verification of Kompakt UV Cabinet
QCL-OP-40 Operation and Performance Verification of Panalytical X-Ray Diffractometer
QCL-OP-41 Operation and Calibration of Halogen Moisture Analyzer Model: HR83, Mettler Toledo
QCL-OP-42 Operation of Milli-Q Gradient Water Purification System ELIX-10
QCL-OP-43 Operation and Performance Verification of Retsch Mixer Miller Model MM 200
QCL-OP-44 Operation and Calibration of Lab India Dissolution Tester
QCL-OP-45 Operation and Calibration of Pierce Reacti-Vap III Evaporator
QCL-OP-46 Operation and Performance Verification of Cooling Incubator
QCL-OP-47 Operation and Performance Verification of HPLC System
QCL-OP-48 Qualification of Analysts in Laboratory
QCL-OP-49 Operation and Performance Verification of Hot Air Oven
QCL-OP-50 Operation and Performance Verification of VK Bio-Dis Extended Release Tester With Dissolution Sampling Station
QCL-OP-51 Operation and Performance Verification of Gas Chromatograph with Headspace Sampler and Auto Injector
QCL-OP-52 Operation and Calibration of Karl Fischer Auto Titrator
QCL-OP-53 Operation and Calibration of Photostability Chamber
QCL-OP-54 Cleaning and Drying of HPLC/GC Vials and Caps
QCL-OP-55 Operation and Calibration of Ultrasonic Bath
QCL-OP-56 Operation and Calibration of Dissolved Oxygen Meter
QCL-OP-57 Handling of Cleaning Validation Samples for Microbial Analysis
QCL-MG Management
QCL-MG-01 Analytical Method Validation
QCL-MG-02 Approval of Artwork
QCL-MG-03 Cross Functional Investigation
QCL-MG-04 Destruction of Samples Sent to Lab for Analysis
QCL-MG-05 Entry into Microbiology Lab
QCL-MG-06 Master formula Record
QCL-MG-07 Preparing for Audit
QCL-MG-08 Reference Working Standards
QCL-MG-09 Sampling and Inspection Procedure for Packing Materials
QCL-MG-10 SOP for Area Colony Test
QCL-MG-11 SOP for Changeover Analysis
QCL-MG-12 SOP for Control Samples
QCL-MG-13 SOP for Fumigation Test
QCL-MG-14 Standardization of Volumetric Solutions and Reagents
QCL-MG-15 Supervision of Analysis
QCL-CB Calibration
QCL-CB-01 Calibration of Glass Measuring Cylinders
QCL-CB-02 Calibration Program
QAS QUALITY ASSURANCE
QAS-001 Cleaning of Dispensing Equipment
QAS-002 Cleaning of Raw, Packing and Finished Goods Stores
QAS-003 Cross Contamination Control
QAS-004 Dehumidifier in Dispensing Room
QAS-005 Dispensing Procedure
QAS-006 Good Documentation Practices
QAS-007 Handling and Storage of Compressed Gas Cylinders
QAS-008 Machine Logbooks
QAS-009 Master Formula Record
QAS-010 Medical Facilities
QAS-011 Packing Material Storage and Issuing
QAS-012 Preventive Maintenance
QAS-013 Production Requirement Sheet
QAS-014 Raw Material Storage and Issuing
QAS-015 Receiving, Storage and Handling of Materials
QAS-016 Rejection or Release of Material
QAS-017 Return Goods Policy
QAS-018 Warehousing of Raw and Packing Material
QAS-019 Submission of Samples and Documents to Regulatory Affairs Department
QAS-020 Investigation of Deficiencies in Raw and Packing Materials After Release by Quality Control
QAS-021 Transfer of Rejected Batch from Production to Finished Goods Warehouse
QAS-022 Receiving, Review, Release and Storage of Analytical Documents, Method Validation / Transfer Documents
QAS-023 Training and Evaluation of QA
QAS-024 Process Validation Program of Tablet Products
QAS-025 Optimizing the Machine Speed of Blister Packing Machine
QAS-026 Preparation, Approval and Control of Record of Analysis / Analysis Report
QAS-027 Responsibilities of Quality Assurance Department
QAS-028 Preparation, Approval, Review and Control of Master Production Records (MPR)
QAS-029 Plant Quality Management Systems Review Meeting
QAS-030 Design and Management of Stability Studies
QAS-031 Monitoring and Cleaning of the Stability Chambers
QAS-032 Performance Verification and Calibration of Sensors of Walk-In Humidity Chamber
QAS-033 Cleaning of Sampling Aids
QAS-034 Precautions for Handling Potent Drug Substances
QAS-035 Handling Inspections by International Regulatory Agencies
QAS-036 Certification of Contract Laboratories and Agencies for Calibration and Validation Services
QAS-037 Operation and Calibration of Sartorius Precision Balance
QAS-038 Reporting, Investigation and Disposition of Incidents
QAS-039 Self Inspection
QAS-040 Packaging of Multiple Semi Finished Batches Into a Single Finished Goods Batch of Same Product
QAS-041 Product Inspection for Physical Defects
QAS-042 Collecting Unit Dose Blend Samples
QAS-043 Evaluation of Effect of Physical Conditions, Temperature on Consignments During Transport
QAS-044 Handling of Product Recalls
QAS-045 Change Control
QAS-046 Vendor Qualification
QAS-047 Investigation of Out-of-Specification (OOS) Results
QAS-048 Annual Product Review
QAS-049 Rounding Rules for Mathematical Calculations
QAS-050 Validation of Standard Cleaning Procedures
QAS-051 Cleaning of Sampling Aids
QAS-052 Master Cleaning Validation Plan, Cleaning Validation Matrix, Protocol and Report
QAS-053 Equipment Equivalency Review and Certification
QAS-054 Operation of Data Logger
QAS-055 Preventive Maintenance of Facility
QAS-056 Cleaning and Sanitation of Toilets
QAS-057 Check List for Facility Maintenance