CONTENTS
Preface
INTRODUCTION
General
Case for Pharmaceutical Industry
ISO 9000 Quality Management System
Perception of Quality
Quality Assurance in the Next Millennium
ORGANIZATION
Structure
Functions
Quality Manual
Site Master File
PERSONNEL
Qualifications
Responsibilities
Hygiene
Training
BUILDING AND FACILITIES
Design and Construction Features
Lighting
Air Handling System
Plumbing
Sewage and Refuse
Washing and toilet Facilities
Sanitation
Maintenance
EQUIPMENT
Equipment Construction
Equipment Cleaning and Maintenance
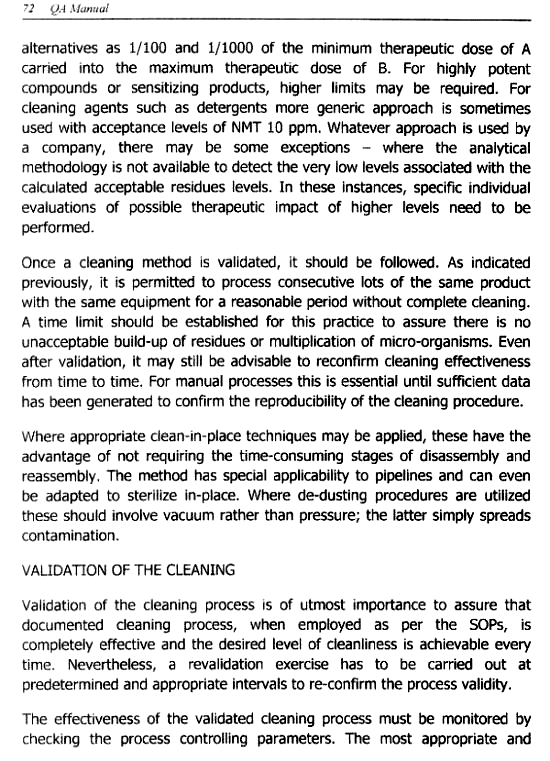
Validation of the Cleaning
Cleaning Schedule
Maintenance
Computer Systems
Validation
Calibration
PRODUCTION CONTROLS
Written Procedures
Change Control
Contamination Control
Sterile Products
Aseptic Process Control
Packaging
WAREHOUSING
Design
Prevention of Cross Contamination
Good Warehousing Practices
Storage
Dispensing
Weighing
LABORATORY CONTROLS
Specification
Raw Materials
Packaging Materials
In-Process Quality Control
Reserve Samples
RETURNED GOODS AND REPROCESSING
Returned Goods
Product Recall
Reprocessing
Rejects/Scrap Disposal
VALIDATION
Applicability
Analytical Test Procedures
Instruments
Equipment Design, Installation and Operation
Facility Design, Installation and Operation
Utility Design, Installation and Operation
Manufacturing Process
Product Development
DOCUMENTATION
General Requirements
Equipment Cleaning and Use Records
Packaging and Labelling Records
Master Production Record
Batch Manufacturing Record
Material Identification
Review of Records
Laboratory Records
Distribution Records
Complaint Files
AUDITS
Internal or External Audits
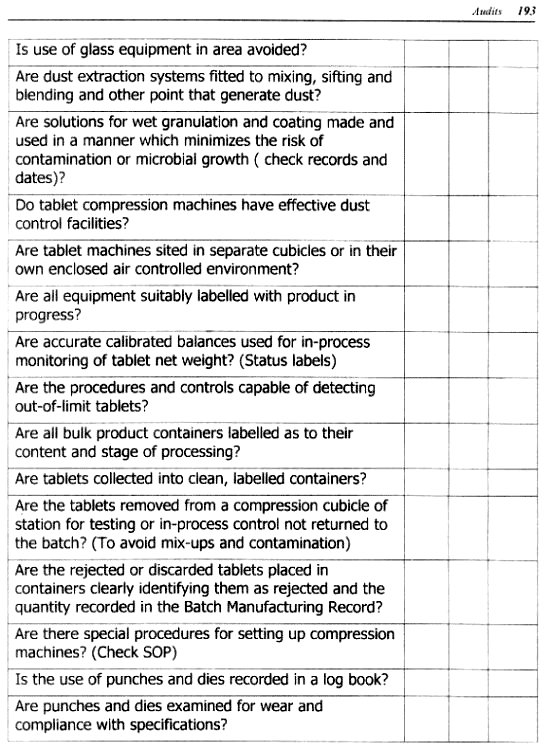
GMP Audits
CONTRACT MANUFACTURING
Contract
Commercial Discussions
Confidentiality Agreements
Technical Agreements
Regulatory Aspects
Validation
Deviations and Change Control
Re-Assessment of Principal Manufacturer
Contract Analysis
BULK PHARMACEUTICAL CHEMICALS
Personnel and Training
Premises and Facilities
Plant and Equipment Documentation
Validation
Impurity Profile
Change control
Material Management
Raw Materials
Containers, Filling and Labelling
Engineering
Quality Assurance and Quality Control
Recovery, Reprocessing and Returns
Storage Life and Stability Testing
Complaints and Recalls
Retention Periods
ANNEXTURE A - Quality Assurance Manual of Good Quality Pharmaceuticals Ltd.
INDEX