CONTENTS
Preface
CARING FOR HEALTH, NATURALLY!
Introduction
The New Ancient Thinking in Medicine
The Rise of Asia: Continental Drift
PharmAsia: Generic Solutions
Herbal Medicine: Aging Roots and Sprouting Branches
China: The Power of Chi
Ayurveda: Healing Trinity
Europe: Doctrine of Signatures
Modern Medicine: Designs from Nature
Better Pill to Swallow: Synthetic Drugs or Herbal?
Food versus Drug Administration of Herbs
Middle Path: Regulating Standards
Blossoming Beyond Borders
NATURE’S PHARMACY
Introduction
Ginseng: Root of Immortality
Gingko: Total Recall?
Snakeroot Plant: The Original Tranquilizer
Bacopa: Memory Plus
Garlic: The Detoxifier
Aswagandha: Root of Rejuvenation
St. John’s Wort: Prozac of plants
Echinacea
Neem: Plant of the 21st century
Shakespearean Herbs: Food Act
Turmeric: Cure with Curry
Ephedra: Mormon Tea
Chocolate: Divine Treat
Beauty Care: Getting Physical
The Blenders Choice
Herbs are Bioreactors
Herbs in Soup: Stirred but not Shaken
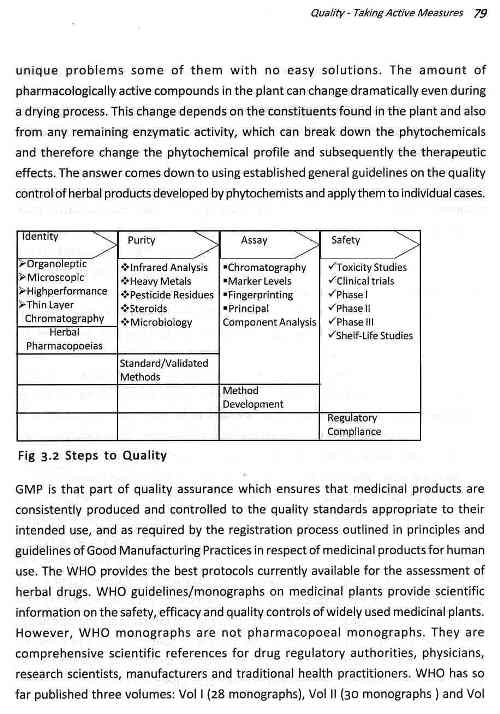
QUALITY - TAKING ACTIVE MEASURES
Standardization: Phases Herbs have to grow Through
Good Agricultural Practices: GAP to Bridge
Identification and Selection: Born Identity
Codex: Phytosanitary Agreement
Good Manufacturing Practices
Good Laboratory Practices
Marking up Herbal Image?
USP Verified
Preclinical Testing
Clinical Trials
Industry Money Skews Drug Overviews?
Pharmacology: Active Site Model
Antioxidants: Radical Thinking
Alkaloid for Lowering Cholesterol
A New Leaf
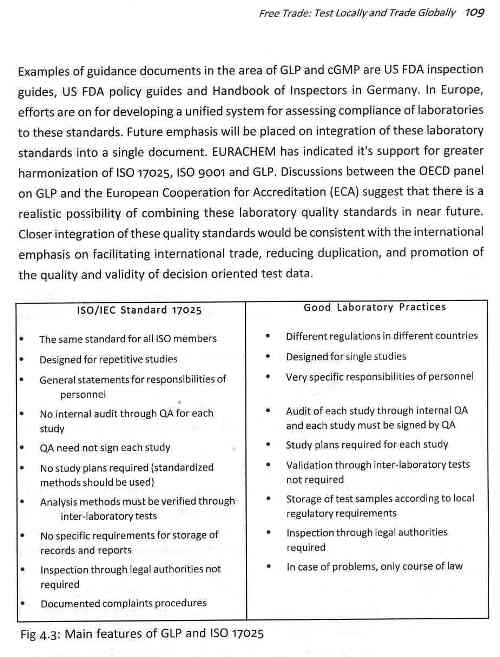
FREE TRADE: TEST LOCALLY AND TRADE GLOBALLY
Introduction
ISO and GXP: Global Standard Practices
Documentation: Living History
Traceability
Calibration
Certified Reference Materials
Validations
Method Selection and Validation
Measurement Uncertainty
Proficiency Testing
Clinical Trials
Double-Blind Studies
The Placebo Effect
Statistical Illusions
Meta-analysis
Mutual Recognition Agreement
FREE TREE AND INTELLECTUAL PROPERTY
Intellectual Property Rights and Wrongs?
Patents: Art of the Possible
Turmeric: Healing Wounds
Neem Campaign
Inca: Jungle Treasures
Aroma of Basmati Patent
Digital Defence: Past Forward
Sharing the Wealth
…and Saving Health
PHARMERS AND FARMACEUTICALS
Drug Discovery Process: New Paradigm
China: Exporting Success
Farming out to CROs
Biodiversity: Clear and Present Danger
Designs on Nature: Farmaceuticals
Herbal Medicines on Tap?
Pharmers: Better the Devil you Grow?
Viva La Vida: Long Live Life
Further Reading
Index