CONTENTS
Preface
Preface to the First Edition
MANAGING PHARMACEUTICAL FACILITIES
Introduction
Role of a Facility Manager
Financial Management
Facilities Condition Assessment (Audit)
Pharmaceutical Facility Validation
Facility Design Qualification
Facility Installation Qualification
Facility Operational Qualification
Utility Systems
Safety for Facility Management
SUSTAINABLE FACILITIES
Ecosystems
Sustainable Manufacturing
Current Scenario
Green Chemistry
Process Mass Intensity
Green Solvents
Green Engineering
Sustainable Buildings
Sustainable Energy Management
Cleanrooms (HVAC)
Facility Lighting
Electric Motors
Compressed Air
Boilers
Sustainable Water Management
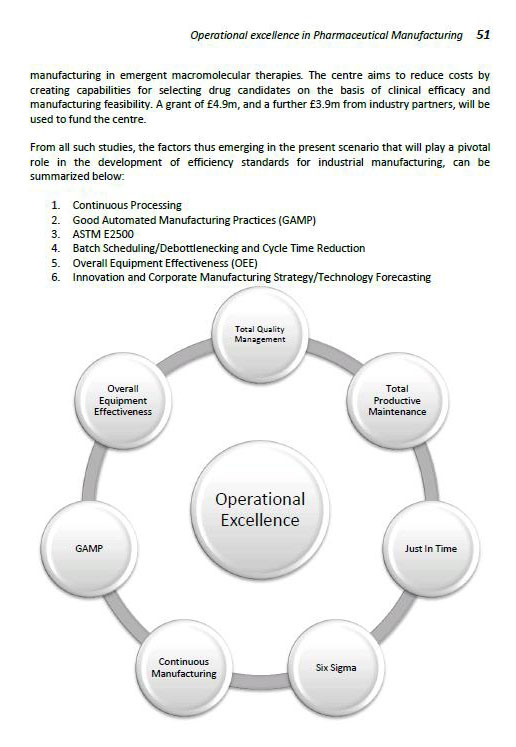
OPERATIONAL EXCELLENCE IN PHARMA MANUFACTURING
Introduction
Focus on Manufacturing Efficiency
Current Efficiency Levels
Continuous Processing of Pharmaceuticals
Automated Manufacturing
Good Automated Manufacturing Practices (GAMP)
ASTM E2500
Optimizing Batch Sizes and Cycle Times
Overall Equipment Effectiveness
Corporate Manufacturing Strategy
PRODUCTION ISSUES
Tablets/Capsules
Introduction
Blenders
Dryers
Tablet and Capsule Equipment
Granulation Mix Analysis
In Process Testing
Process Notes
Common Problems in Tabletting
Liquid Orals
Process Notes
Topical Preparations
Potency Uniformity
Equipment and Production Control
Preservatives Used in Topical Preparations
Preservatives Used in Ophthalmic Preparations
Pharmaceutical Water Systems
System Validation
Microbial Limits
Water for Injection Systems
Purified Water Systems
EQUIPMENT CLEANING AND MAINTENANCE
Introduction
Maintenance Methodologies
Lean Maintenance
Calibration of Equipment
Procedure
Calibration Program
Calibration Standards
Calibration Procedures
Instrument Maintenance Records
Calibration Instruments
Electric Motors
Basic guidelines
Motor Repair / Replacement Decisions
Economic Comparison
Centrifugal Pumps
Fluid Bed Dryer
Mass Mixer
Multimill
Planetary Mixer
High Shear Mixer Granulator (Rapid Mixer Granulator)
Sifter
Tray Dryer
Wurster Coater Dryer
Double Cone Blender
Tablet Compression Machine
Inspection and Maintenance of Compression Tooling
Common Problems Encountered with Tablet Tooling
Vibratory Tablet De-Duster
Conventional Coating Pan
Polishing Pan
Strip Sealing Machine
Capsule Autoloader
Capsule Inspection Cum Polishing Machine
Dry Syrup Powder Filling Machine
Capsule Filling Machine (Manual)
Capsule Filling Machine (Semi-Automatic)
Tanks, Accessories, Utensils And Stirrers
Filter Press
Liquid Oral Filling Machine
Bottle Labeling Machine
Component Preparation Tank
Melting Tank
Ointment Mixer (Silverson Type)
Ointment Bulk Storage Drum
Tube Filling Machine
Equipment For Sterile Products (General)
Sterile Product Vessels
Stirrer
Vials /Ampoules Washing Machine
Rubber Stopper Washing Machine
Membrane Filter Holder
Filtration Tubing
Ampoule Filling And Sealing Machine
Vial /Ampoule Filling Assemblies And Accessories
Autoclave
Preventive Maintenance
Vial Sealing Machine
Vial/Ampoule Inspection Machine
Compressed Air
Boiler
The Boiler Room
Piping Systems
Steam Traps
Water Treatment
Pumps
The Venting System
The Boiler
Test Firing
HVAC/R
Fans
Air-Handling Unit
Chillers and Condensers
Cooling Towers
Ducts Cleaning
Clean Room Monitoring
Safety Tips for HVAC Maintenance
Pharmaceutical Water
Cleaning of Piping
Passivation
Sanitization
Setting up a Machine Shop
Guidelines and Suggestions
Safety
Maintenance
Regulatory and Compliance Issues
COMPUTERIZED MAINTENANCE MANAGEMENT SYSTEM
Introduction
Computerized Maintenance Management Systems (CMMS)
Advantages of CMMS
Guidelines for Small Companies
Implementation of CMMS
Implementation Basics
Framework for Transition
Preventive Maintenance
Continuous Process
Automatic Data Collection
Elements of CMMS
Work Order
Assets
Location
Preventive
Employee
Masters
Reports
Work Orders
How to Open a New Work Order
Filter Out Work Order Information
Close a Work Order
Add Labour in the Work Order
Direct Issue in a Work Order
View Total Cost of a Work Order
Assets
Add a New Asset
How to Filter out Assets Information
Add a New Location
Filter Out Location Information
Employee/Requester
Add a New Employee/Requester
Filter Out Employee/Requester Information
Preventive Maintenance
Register a New PM Task
Filter Preventive Maintenance Schedules
Generate PM Work Orders
Masters
Department
Failure Code
Assets Category
Suppliers/Contractors
Reports
WORK ENVIRONMENT AND SAFETY
Housekeeping
Introduction
Capsule Department
Tablet Department
Liquid Oral Department
Ointment Department
Sterile Area
Raw, Packing and Finished Goods Stores
Pest Control And Disinfection
Managing Older Buildings
Roofing Maintenance
Indoor Air Quality
Disaster Management
Planning For an Emergency
Damage Control
Safety
Safety Audits
Pharmaceutical Hazardous Areas
Safety Procedures
Safety Management through Air Handling Systems
Fire Detection and Control
Hazards Specific to Pharmaceutical Industry
Pharmaceutical Manufacturing Precautions
Personal Protective Equipment
Electrical Safety
Electrical Safety Regulations and Standards
Electrical Safety Program
Electrical Hazards
Work Procedures, Tools and PPE
Safety by Design
First Aid
Facility Security
TRAINING
Introduction
Writing a Training Program
Structure of Training Programs
Conducting Training Sessions
Class
Objectives
Material
Props
Lesson Plan
Environment
Training Pointers
Tips To Become a Better Trainer
Presentation Skills
Body Language
The Role of Questions
Assessment
Documentation of Training
A Case Study
Sample Training Programs
Training Program for Supervisors
Training Program for Workmen
Training Program for Clean Room
Training Program for Autoclave
Training Program for Dry Heat Sterilizer
Training Program for Janitors
Training Program for Laminar Air-Flow
Training Program for Membrane Filter Holder
Training Program for Personal Hygiene
Training Program for Production Supervisor
Training Program for Sterile Stoppering
Training Program for Workmen
VALIDATION
Introduction
Purpose of Validation and Qualification
Responsibility for Validation and Qualification
Validation Master Plan
Purpose
Format and Content
Installation and Operational Qualification
Introduction
Installation Qualification (lQ)
Operational Qualification (OQ)
Re-Qualification
Non-Sterile Process Validation
Introduction
Prospective Validation
Concurrent Validation
Retrospective Validation
Re-validation
Change Control
Cleaning Validation
Introduction
Documentation
Sample Formats
Format for an installation qualification protocol
Format for an operational qualification protocol
Format for a performance qualification protocol
Examples of IQ, OQ, and PQ Protocols
Content Requirements for Equipment/Systems
Water for Injection
Temperature Controlled Equipment
Centrifuges
Blenders, mixers and homogenizers
Pumps
Backup Power Generator
Controlled Air Equipment
Measuring Apparatus
Filter for Integrity Testing Apparatus
Format for a Process Validation Protocol
Validation Protocols
ENVIRONMENTAL MONITORING
Introduction
Pharmaceutical Products
Botanicals
Pharmaceutical Preparations
Diagnostic Substances
Biological Products
Industrial Processes In The Pharmaceutical Industry
Research and Development
Production of Bulk Pharmaceutical Chemicals
Chemical Synthesis
Natural Product Extraction
Fermentation
Formulation into Final Dosage Forms
Raw Material Inputs And Pollutant Outputs
Air Pollution
Bulk Manufacturing
Formulation
Air Pollution Control Equipment
Condensers
Scrubbers
Combustion or Incineration
Adsorption
Wastewater
Solid Wastes
Pollution Prevention
Material Substitutions
Process Modifications
Good Operating Practices
REGULATORY INSPECTIONS
Introduction
Trends In Regulatory Inspections
Managing Regulatory Inspections
SOP for Regulatory Inspections
Staff Training for Inspection
What Inspectors look for in a GMP Audit
Good Practices for Hosting Regulatory Inspections
Inspections of Foreign Pharmaceutical Manufacturers
Inspection of Quality Systems/GMP Audit
Records
Organization and Personnel
Buildings and Facilities
Equipment
Storage of Raw and Packing Materials
Production and Process Controls
Critical Manufacturing Steps
Equipment Identification
In-Process Testing
Packaging and Labeling
Laboratory Controls
Control Records
Returned Drug Products
Inspection Of Tablet/Capsule Section
Raw Material Receipt and Granulation
Tablet Compression and Coating/Capsule Filling
Inspection of Liquid Orals Section
Equipment
Compounding
Packaging
Inspection of Topical Products Section
Manufacturing
Filling and Packaging
Cleaning
Microbiological Controls (Non-Sterile Topicals)
Inspection of Sterile Products
Facilities
Environment
Equipment
Water for Injection
Sterilization
Packaging
Personnel Practices
Inspections of Quality Control Laboratories
Out-of-Specification (OOS) Laboratory Results
Laboratory Errors
Laboratory Investigations
Formal Investigations
Investigation Documentation
Product Failures
Retesting
Resampling
Averaging Results of Analysis
Microbiological
Laboratory Records and Documentation
Laboratory Standard Solutions
Methods Validation
Equipment
In Process Controls and Specifications
Stability
Computerized Laboratory Data Acquisition Systems
Laboratory Management
Inspection of Utilities
HVAC
Pharmaceutical Water System
Pharmaceutical Steam Systems
Compressed Air
References
Reference Data for Plant Managers
Index